Abstract
Background: In Hodgkin Lymphoma (cHL) tissues a few neoplastic cells are embedded in an overwhelming mixture of inflammatory and immune cells. These latter contribute to tumor expansion by providing pro-survival signals and favoring immune escape. Current staging system categorizes patients in early and advanced stages reflecting tumor burden and dissemination. Overall, up to one-third of patients may recur after induction treatment and no robust biomarkers have been yet validated to identify progressors. Despite the prominence of the inflammatory immune microenvironment, the play of some key immunomodulators such as myeloid type-1 (mDC1), type-2 (mDC2) and plasmacytoid (pDC) dendritic cells (DCs) in the cellular cross-talk underlying cHL is largely unexplored.
Methods: We assessed blood DCs levels in 54 newly diagnosed patients and 29 age-matched healthy controls through multi-parametric flow cytometry. The Blood Dendritic Cell Enumeration Kit from Miltenyi Biotec (Bergisch-Gladbach, Germany) was used, with 0.27 (r,0.09-0.42), 0.02 (r,0-0.04), 0.19 (r,0.09-0.37) as reference median rates for mDC1, mDC2 and pDC, respectively. Mann-Whitney, Wilcoxon sum rank, and log-rank two-sided tests were used as appropriate with p<0.05 regarded as significant.
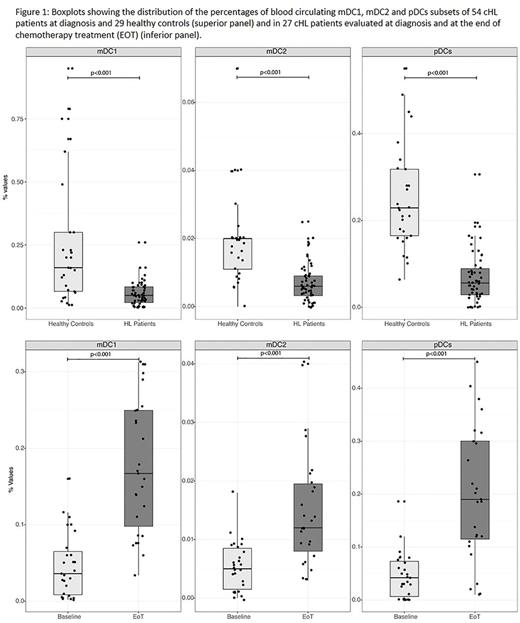
Results: The median age was 34 years (r,19-72); 23 patients (43%) had early stage [i.e. I, IIA/B, IIA extranodal (E) or bulky (X)], 31 (57%) advanced stage (i.e. IIB E or X, III and IV). B-symptoms affected 25 patients (46%) while bulky was present in 24 (44 %). Among the 23 patients with early stage, 48% had 3 or more nodal sites according to GHSG criteria while 45% of those with advanced stage had International Prognostic Score (IPS) 3 to 7. Fifty-two patients received ABVD regimen; Overall, 49 achieved a negative fluorodeoxyglucose positron emission tomography scan after the first two cycles (PET2). At a median f.u. of 33.5 mo.s, one primary progression and three relapses (<12 months: N=1; >12 months: N=2) occurred. All DCs subsets had lower frequencies in cHL patients than in healthy controls (p<0.001) (Figure 1; upper panel). In detail, in the control group, the relative median counts (25th; 75th) were 0.160 (0.06;0.3) for mDC1, 0.020 (0.01;0.02) for mDC2, 0.230 (0.16;0.32) for pDC. In the 54 cHL patients, the relative median counts (25th; 75th) were 0.050 (0.02;0.09) for mDC1, 0.006 (0.003; 0.009) for mDC2, 0.056 (0.028; 0.092) for pDC. The median rates were significantly lower for the advanced vs early stage, for both mDC1 (0.032 vs 0.070; p=0.008) and mDC2 (0.005 vs 0.009; p=0.001) subsets. Also, the median mDC2 counts were reduced in the case of bulky (0.004 vs 0.008; p=0.001) and extranodal (0.005 vs 0.006; p=0.019) disease. Patients with B symptoms had lower levels for mDC1s (p=0.029), mDC2s (p=0.005) and pDCs (p=0.020). At the end of treatment (EOT) 27 patients were reassessed: median (25th;75th) fractions of mDC1, mDC2 and pDC subsets raised from 0.036 to 0.167 (0.086; 0.250)(p< 0.001), from 0.005 to 0.012 (0.007; 0.020)(p< 0.001), from 0.042 to 0.190 (0.110; 0.300)(p< 0.001), respectively (Figure 1, lower panel). Such increases (4.6 fold for mDC1, 2.4 for mDC2, 4.5 for pDC) fully aligned circulating DCs subsets levels with those of interquartile ranges of healthy controls. EOT recovery of DCs was independent from any of the usual negative prognosticators/predictors, including IPS 3-7 and positive PET2. At a median f.u. of 35 months, PFS for the 31 patients with advanced stage showed a trend in favor of those with mDC2 levels above the median (100 vs 73%; p=0.06).
Conclusions: Patients with cHL display a constitutive abatement of circulating DCs that correlated with tumor burden and dissemination (mDC1 and mDC2 subsets) and/or disease activity (pDC subset). Notably, treatment completion and response achievement were associated with a statistically significant reconstitution of circulating DCs pool into line with interquartile ranges of healthy controls. Recovery was unaffected by the presence of negative prognosticators. While patients with more compromised mDC2 levels showed a wider anatomical extent of disease and a trend towards a worse survival, analysis of larger cohorts is warranted to ascertain the prognostic impact of this finding conclusively. Results suggest that DCs may contribute to the disturbed immunological interplay cells typical of cHL and prompt a further evaluation of their value as a potential new biomarker.
Pinto: Roche: Honoraria; Merck Sharp Dome: Honoraria; Celgene: Honoraria; Bristol Myers Squibb: Honoraria; Gilead: Honoraria; Millenium Takeda: Research Funding; Helssin: Honoraria; Mundipharma EDO: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.